Executive Summary
On May 8, 2019, the Trump administration announced that the Centers for Medicare & Medicaid Services (CMS) would require drugs covered by Medicare or Medicaid to include a list price in any DTC television commercial within 60 days of the announcement, if their list price is at least $35 for a month’s supply or a course of treatment. The ruling is virtually the same as the one proposed in October 2018, and only products that advertise on TV are affected directly by the ruling.
This POV provides a brief summary of the background to this ruling, a summary of its specifics, some reactions to it, and our recommendations to clients in its wake.
Background
The requirement to include list prices in DTC ads was first proposed in October 2018 in the “American Patients First” blueprint released by HHS Secretary Azar.
From October 2018 through May 2019, industry group PhRMA and individual corporations responded in two ways.
First, they offered a different viewpoint to the logic behind the blueprint, pointing out that publishing list prices would be unlikely to accurately reflect the actual price a patient would pay, and could therefore confuse patients, which might cause them to erroneously avoid medication that could help them.
Second, PhRMA released updated DTC Principles, the organization’s voluntary guidelines for advertising. Some companies did begin to choose to include links to pricing information, or the information itself, in their ads.
Meantime, some pharmaceutical companies have begun to list drug prices, or informational websites, in their commercials in advance of this ruling. Industry trade group PhRMA launched an update to their voluntary DTC principles, to include information about pricing, and on May 8, 2019, also announced the launch of MedicineAssistanceTool.org, a search engine for patient-assistance programs.
For additional background, see our Oct. 2018 POV, Sharing Drug Pricing With Consumers: An Intouch Primer.
Specifics
The recent rule can be read in its entirety here.
What: Prescription drugs or biologics that are reimbursed by Medicaid or Medicare — if they have a list price of at least $35 for a 30-day supply or typical course of treatment — must include a textual statement at the end of the advertisement. It was not mandated that voiceovers include the pricing statement.
Where: In television advertisements (including broadcast, cable, streaming, and satellite). It was not mandated that YouTube channels or other videos include the pricing statement.
When: Effective July 9, 2019
Also: Manufacturers are permitted to include an up-to-date list price of a competitor’s product, so long as they do so in a truthful, non-misleading way.
To see the information CMS says must be included in a television ad, see page 5 here.
Reactions – Media
As noted, industry has questioned the premise of the ruling and whether it will be beneficial for patients.
Some media also question another premise of the ruling – to wit, whether it will result in lower prices. NPR noted, “Secretary of Health and Human Services Alex Azar said today that when it comes to changing prescription drug prices, ‘putting prices in TV ads may be the most significant single step any administration has ever taken.’ But patient advocates are not convinced it will have an immediate impact on drug pricing.”
Others, like LA Times business columnist Alex Lazarus, were skeptical of the regulation’s efficacy generally. In a column titled “Drugmakers need to take a chill pill over new price-disclosure requirement,” Lazar opined, “This indicates that Trump’s commitment to patient welfare is little more than political posturing.”
Reactions – Industry
Meanwhile, the industry has begun taking action. As noted above, PhRMA’s voluntary DTC Principles were updated in October 2018 to include the following 19th principle:
“All DTC television advertising that identifies a medicine by name should include direction as to where patients can find information about the cost of the medicine, such as a company-developed website, including the list price and average, estimated, or typical patient out-of-pocket costs, or other context about the potential cost of the medicine.”
To that end, on January 8, Eli Lilly began running ads for Trulicity that directed viewers to a website that gave its list price and average out-of-pocket costs, and information on patient-assistance programs. That website, lillypricinginfo.org, now includes information for five Lilly brands: Emgality, Olumiant, Taltz, Trulicity, and Verzenio.
On February 7, Johnson & Johnson announced that its TV ads for Janssen’s Xarelto would begin to include the drug’s list price and typical out-of-pocket costs in the ad, and direct them to the brand’s website for further information. On March 29, the ad first aired, making Xarelto the first drug to include the price in its ad.
On May 8, PhRMA issued the following statement:
We are concerned that the administration’s rule requiring list prices in direct-to-consumer (DTC) television advertising could be confusing for patients and may discourage them from seeking needed medical care. We support providing patients with more transparency about medicine costs, which is why our member companies voluntarily began directing patients to links to comprehensive cost information in their DTC television advertising. After speaking with patients across the country, we learned that patients prefer this approach.
This is also why today we announced the launch of a new platform for patients, caregivers and health care providers called the Medicine Assistance Tool, or MAT. This tool links to the websites referenced in company DTC television advertising and includes a search tool to help patients connect to financial assistance programs. This effort is just one of several ways our members are working to ensure patients have the information they need to make more informed health care decisions.
While we are still reviewing the administration’s rule, we believe there are operational challenges, particularly the 60-day implementation timeframe, and think the final rule raises First Amendment and statutory concerns.
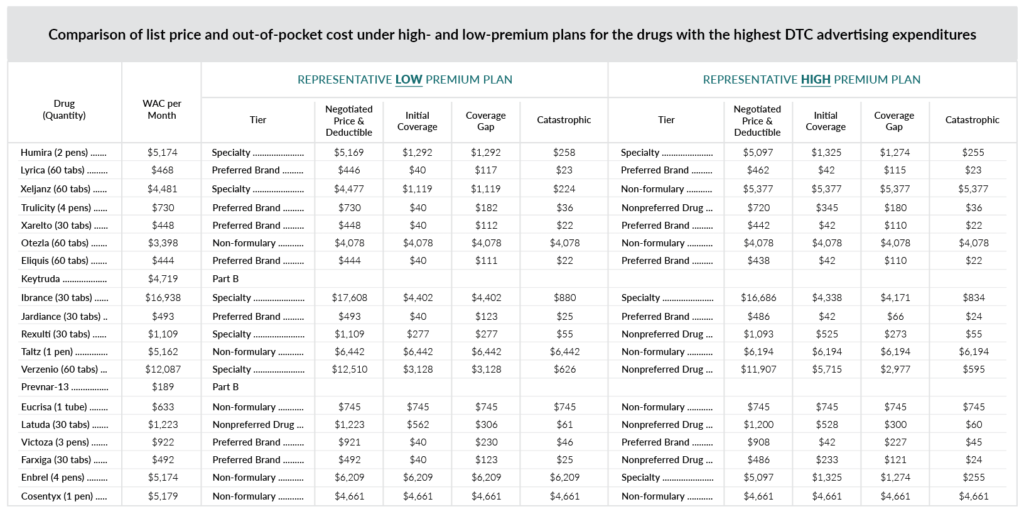
In its Friday, May 10 edition, the Federal Register included a price-comparison chart showing list prices and out-of-pocket costs under high- and low-premium plans. Intouch’s reformatted version is shown below; you can see the original here.
Industry think tank Coalition for Healthcare Communications (CHC) issued a statement communicating a similar level of disappointment, but stopped short of promising legal action:
It is unfortunate that HHS moved forward on this plan, especially given the constructive alternatives PhRMA and individual pharmaceutical companies have put forward to provide accurate pricing information in context.
CHC also points out the unusual method laid out for enforcement:
Another interesting aspect is that this regulation will not be enforced directly by CMS or the FDA’s Office of Prescription Drug Promotion (OPDP). Instead, HHS will “maintain a public list” of drugs with advertisements that do not comply, and will rely on competing biopharma companies to take private legal action against one another under the Lanham Act for unfair competition. One wonders whether any pharmaceutical company would take such action or, if they did, be able to prove they were harmed.
Recommendations
While the CMS ruling dictates what pricing information must be included in television spots, it’s been left up to the manufacturers to provide important clarity around that pricing information. The opportunity exists to provide a realistic context around the list price statement, either in the TV spot or in an accompanying website, that can ensure they are able to inform patients in a helpful way. As in the Janssen ad for Xarelto, this can be done by providing estimates of average out-of-pocket costs.
Some companies may choose to take a wait-and-see approach, but they should be prepared to come into compliance if required. This could simply mean pulling noncompliant TV spots at such time as that becomes necessary. Whether a brand chooses to immediately act or to hold off, though, it’s important to recognize this opportunity to demonstrate patient centricity and to consider additional avenues for doing so.
PhRMA’s MedicineAssistanceTool.org is one such step toward that end. Additional tools, such as online co-pay lookups, or coverage checkers like this one from Ozempic, are ways that brands can help patients with even more specificity. These can also include information about patient-assistance programs and third-party foundations that can assist patients.
Advertisers can provide pricing clarification in additional channels such as point-of-care and print advertising. Brands should also consider ramping up digital advertising pointing to contextual resources, particularly in markets where television ads are running.
Sales reps can ensure that HCPs have accurate information on ultimate patient costs through interactive visual aids, tools, and ongoing marketing messaging that not only educate the HCP, but also assist in clarifications for patients who might be confused about what they saw on television.
Finally, brands should deploy mechanisms to gather information to understand pain points and identify opportunities for improvement. Sales reps and call centers should report the volume and nature of inquiries and concerns as it relates to pricing.
Conclusion
Pharmaceutical pricing has been questioned by regulators for more than half a century. While the industry is clearly moving toward greater pricing transparency, this latest ruling may not have the results its proponents hope for.
As the industry has noted in public commentary, in PhRMA’s response, and in the comments to the draft rule, the major concern expressed is the risk of confusion to consumers. A list price, without the discounts, rebates, and co-pays specific to each patient’s coverage situation, does not tell a patient what they need to know: how much they will pay at the pharmacy counter.
It’s easy to see how the potential result – HCPs and patients making treatment decisions based on erroneous assumptions – could be bad for patients in a variety of ways. Patients could avoid seeking care altogether, thinking that help is beyond them financially. Or they might fall prey to a “value bias” – choosing the priciest medication available, thinking expensive must equal better.
While the rule did include responses to the legal challenges made by public comments, we do expect legal challenges to arise and continue. PhRMA’s May 8 response says that they “are still reviewing the administration’s rule,” which does not rule out litigation. Challenges based upon the First Amendment and the question of “compelled speech” are likely.
For assistance in ensuring PhRMA and CMS compliance across your DTC advertising, or to discuss effective, customer-friendly options for pricing transparency, contact your Intouch representative or Brady Walcott, Executive Vice President, Intouch Group at brady.walcott@intouchg.com. For help with strategic considerations surrounding longer-term pricing and contracting, contact Mike Motto, SVP Market Access, Intouch Group, at mike.motto@intouchsol.com.
©Intouch Group 2018
Intouch Group is not a law firm, and nothing in this advisory should be construed as offering legal advice or counsel.

